Sedlak
Blog
Sedlak 2025 Virtual Lunch & Learn Events: Optimizing Your Supply Chain
Did you miss our virtual series this year on Optimizing Your Supply Chain? If so, you can now explore our virtual event recaps and recordings!
Continue ReadingTariff Blog Series: Exploring Alternatives to Traditional Sorters
By Daniel Hyla & Dave Teeple Distribution centers have long relied on traditional sortation systems such as tilt tray and cross-belt sorters. These technologies, first developed in the mid-20th century, were designed to automate the high-volume sorting of uniform items into specific destinations.
Continue ReadingTariff Blog Series: Tax Credit Opportunities Hidden in Your Supply Chain
By Daniel Hyla & David Teeple The Research & Development (R&D) Tax Credit has been a cornerstone of U.S.
Continue ReadingTariffs Blog Series: Navigating the Future of Inventory Management – A 2025 Playbook
By Daniel Hyla and David Teeple Inventory Management often sits at the crossroads of success and failure in today’s volatile supply chain. When it is well managed, it enables organizations to meet customer demand, maintain service levels, and control costs.
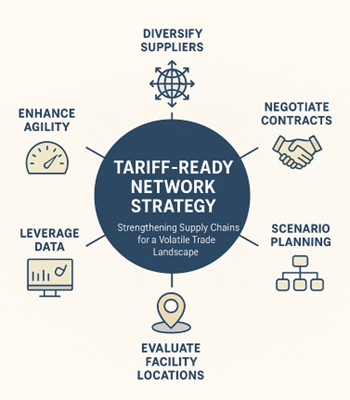
Continue ReadingNavigating the Tariff Storm Part XIV: Rethinking Network Strategy to Navigate Global Tariff Disruptions
By Daniel Hyla and Michael Davis Tariffs have continued to disrupt global trade, paralyzing many supply chains. As costs rise and uncertainty persists, networks are under scrutiny as organizations search for ways to minimize impact.
Continue ReadingNavigating the Tariff Storm Part XIII: Rethinking Your Investment Strategy – CapEx vs. OpEx
By David Teeple & Daniel Hyla Tariffs have disrupted both short-term and long-term investment strategies across industries, with uncertainty surrounding future trade policy continuing to weigh heavily on executive decision-making. While recent trade agreements have helped reduce some of the ambiguity, the full economic impact of tariffs has yet to materialize.
Continue ReadingNavigating the Tariff Storm Part XII: Don’t Let Evolving Tariffs Distract You from Making Operational Improvements
By Daniel Hyla and David Teeple Tariffs have been rewriting the rules of global trade, disrupting supply chains for much of 2025. While initial projections forecasted an economic downturn following the rollout of these tariffs, the economy has continued to defy expectations, growing at an annualized rate of 3% in Q2, outpacing the forecasted 2%1.
Continue ReadingNavigating the Tariff Storm Part XI: Light at the End of the Trade Tunnel?
By Daniel Hyla & David Teeple Trade deals with Japan, Indonesia, and the Philippines were finalized this week, marking a continued step forward in the U.S.
Continue ReadingNavigating the Tariff Storm Part X: Strategic Tariff Tools for Resilient and Agile Supply Chains
By Daniel Hyla and David Teeple Tariffs continue to create waves of disruption across the global economy. In recent weeks, the United States has imposed new tariffs ranging from 10% to 50% on imports from over 20 countries, including Canada, Brazil, and key Asian exporters.
Continue ReadingNavigating the Tariff Storm Part IX: How the Global Trade War is Heating Up
By Daniel Hyla and David Teeple The tariff-related winds picked up momentum this week as the Trump administration issued formal letters to countries that have yet to finalize trade agreements with the United States. On Wednesday, July 9th, the expiration date for the reciprocal tariffs imposed on April 2nd — known as “Liberation Day” — […]
Continue ReadingNavigating the Tariff Storm Part VIII: Is Your Supply Chain Ready?
By Daniel Hyla and David Teeple Tariffs are no longer just a talking point among supply chain professionals — they’ve become a global headline. Over the past few months, businesses across all industries have scrambled to respond, forming dedicated tariff mitigation teams, re-evaluating sourcing strategies, and defining new approaches to increase agility and transparency across […]
Continue ReadingNavigating the Tariff Storm Part VII: Trade Policy Update
By Daniel Hyla & David Teeple Tariff-related agreements have been overshadowed by the evolving Israel-Iran conflict, which has had the full attention of the Administration. Despite this, the United States finalized a new trade agreement with the United Kingdom on June 16th—one that expands market access for U.
Continue ReadingNavigating the Tariff Storm Part VI: The Good, The Bad, & What’s Next
By Daniel Hyla & David Teeple Introduction Since the Trump administration declared “Liberation Day” in early April, signaling a sharp pivot in trade policy, tariffs have been disrupting global trade. The impact was immediate.
Continue ReadingNavigating the Tariff Storm Part V: Tariffs at the Table
By Dave Teeple and Daniel Hyla Navigating the Tariff Storm Part I Navigating the Tariff Storm Part II Navigating the Tariff Storm Part III Navigating the Tariff Storm Part IV Tariffs are steadily eating away at the patience of both businesses and consumers, as policy uncertainty continues to cloud the economic outlook. Economists have recently […]
Continue ReadingKey Considerations for Implementing a Goods-to-Person (GTP) System
In the fast-evolving world of distribution and fulfillment, Goods-to-Person (GTP) systems have emerged as a game-changing solution. These systems bring items directly to workers, significantly improving efficiency, accuracy, and ergonomics.
Continue ReadingNavigating the Tariff Storm Part IV: How Mid-Size Retailers Can Stay Afloat
By David Teeple and Jake Snider Navigating the Tariff Storm Part I Navigating the Tariff Storm Part II Navigating the Tariff Storm Part III The year 2025 has ushered in a challenging new era for mid-size retailers across the United States. A fresh wave of tariffs, coupled with ongoing global trade tensions, is fundamentally reshaping […]
Continue ReadingNavigating the Tariff Storm Part III: Surviving Tariff Turbulence – What to Do Now
By David Teeple and Daniel Hyla Navigating the Tariff Storm Part I Navigating the Tariff Storm Part II Since April, shifts in global tariff policy have sent disruptive ripples through supply chains. Companies are now beginning to feel the effects — quarterly earnings are taking hits, customers are being warned of impending price increases, and […]
Continue ReadingNavigating the Tariff Storm Part II: How 2025 Tariffs Are Reshaping Strained Global Supply Chains
By David Teeple & Daniel Hyla Global supply chains, while perhaps slightly less pressured than the peak crisis years (2021-2022), remain complex and vulnerable to disruptions (geopolitics, labor issues, weather). The Global Supply Chain Pressure Index (GSCPI) is easing, but challenges persist.
Continue ReadingFacility Design Considerations for an Expanded or New Distribution Center
Whether you are planning an expansion or a new distribution center with any level of Material Handling Equipment (MHE) and / or automation, there are many design factors to consider— way too many for a blog, so we’re going to focus on the biggies. Size: Start by selecting the storage and handling concepts you will […]
Continue ReadingNavigating the Tariff Storm Part I: Ways to Mitigate the Impact of Tariffs
With the uncertainty of how the tariffs will impact your business, it is critical to implement a mitigation strategy. Regardless of the results of the tariff negotiations, the following actions can position your supply chain for success.
Continue ReadingAchieve a Competitive Advantage with Supply Chain Design – A Sedlak Virtual Lunch & Learn with Optilogic
Summary: The session focused on enhancing competitive advantage through effective supply chain design, featuring insights from Sedlak Management Consultants and Optilogic. Lou Cerny, Vice President, welcomed participants to Sedlak’s fifth annual Lunch and Learn series.
Continue ReadingUtilize Supply Chain Modeling to Answer the Question “Where Do We Go from Here?”
Whether you have a single distribution facility or multiple facilities in your network, supply chain rationalization and modeling provide significant insight and identify opportunities to optimize your supply chain and customer service. Today’s robust modeling software can process the large data files required to develop a baseline, down to the SKU-level, and then run the […]
Continue ReadingBig Data and Your Organization: Where Do We Go Next?
By Brian Donnelly As the Ecommerce, Warehousing, and Supply Chain sectors have grown over the past decades, the need for data and data solutions has become a central focus for many organizations. They face daily challenges and seek answers to questions like: Should we construct a new facility?
Continue ReadingSupply Chain Cybersecurity for Material Handling & Logistics Systems
By Brian Van Vleet, Rockwell Automation With the increasing adoption of AI, robotics and automation, modern control systems of sortation and processing equipment are in the crosshairs of highly motivated individuals who exploit vulnerabilities in computer systems, networks and software for financial or political gain. Distribution and fulfillment operations need an especially heightened awareness due […]
Continue ReadingSedlak Attends MODEX 2024
A team of Sedlak consultants recently attended MODEX, a leading conference for manufacturing and supply chain professionals that focuses on innovation, technology and networking. The team that attended included David Teeple and Michael Davis, both Directors of Client Services; Ryan Prahler, Client Executive; Rob Bober, Manager; and Brian Donnelly, Senior Consultant.
Continue ReadingDave Teeple on How to Implement New Technology in Fulfillment Operations
In this episode of the Unboxing Fulfillment podcast, host Harry Drajpuch interviews David Teeple, Director of Client Services at Sedlak Supply Chain Consultants. Sedlak is a comprehensive supply chain and distribution logistics advisory firm that has been in business since 1958.
Continue ReadingAutomation in the Supply Chain from Processes to Robotics: Tecsys and Sedlak
As a supply chain professional, you need to better understand how current automation movements can impact your business. Automation in the Supply Chain from Processes to Robotics, which is a recorded session from Sedlak’s 2023 virtual event series, Balancing People, Systems and Automation, looks at which processes are being automated within the supply chain, as […]
Continue ReadingSpotlight on Robotics
If you went to ProMat in Chicago this spring, you saw hundreds of robots performing distribution tasks including storage, retrieval, pick assist and sortation. At Sedlak, we are fortunate to be brought up to speed on these technologies on a regular basis when vendors schedule a lunch hour with us to demonstrate their offerings and […]
Continue ReadingOptimizing the Balance Between People, Information Systems and Automation
It was great to see that ProMat was so well attended this year! After the past few years it was good to reconnect with friends and colleagues.
Continue ReadingCongratulations to Our Newly Promoted Associates!
It is our pleasure to announce the following promotions and congratulate each of these Sedlak associates on their achievements! Rob Bober, Manager.
Continue ReadingSedlak’s Lunch & Learn Program for Suppliers
Sedlak maintains relationships with a number of key suppliers, which include integrators, third party logistics providers, and providers of material handling equipment and storage media, information technology, robotics, real estate, and construction. We work with these suppliers in order to keep our staff abreast of vendor capabilities, product offerings, industry news, and to obtain competitive […]
Continue ReadingFull Automation: 6 River Systems & Sedlak
Automation is rapidly becoming essential, not optional, in addressing today’s supply chain challenges. While the main focus of automation has always been on optimizing the picking function, where the majority of labor is required, there are opportunities to utilize AMRs and other automation technologies to improve performance throughout an operation, including replenishment, Value Added Services […]
Continue ReadingPerformance Optimization Through Data Analytics: Sedlak, Easy Metrics and Ferguson
Having critical financial and operational data will ensure that your company has the data necessary to operate as effectively as possible. That’s why Easy Metrics developed their OpsFM (Operations Financial Management) platform.
Continue ReadingGTP – Optimizing Fulfillment Capabilities: Swisslog, Ahold Delhaize and Sedlak
GTP, or Goods to Person, is a different way of thinking about warehouse picking that moves items to workers rather than workers to items. It cuts down on fatigue, mistakes, and overhead.
Continue ReadingAgile Material Handling with AGVs: Dematic, McCormick & Sedlak
Automated Guided Vehicles (AGVs) have been around for decades, but only in recent years have they become a viable, effective solution for supply chains. Over the last 10-15 years there have been significant changes in the capabilities of AGV offerings.
Continue ReadingSupply Chain Agility Through the Use of Financial and Labor Management Software (Part 3 of 3)
Blogs 1 and 2 (see the bottom of this post for links) of this series primarily focused on longer term options to improve overall supply chain agility through Network Optimization Modeling and the incorporation of Automation. Unfortunately, with few exceptions such as acquiring AMRs, these options can take 12-24 months to implement to provide the […]
Continue ReadingSupply Chain Agility: Dealing with the Challenges of Living in Interesting Times (Part 2 of 3)
As noted in my previous blog, the phrase “May you live in interesting times” can be viewed as a blessing or a curse. Regardless of how it is interpreted, we have no choice but to deal with the challenging times we are living in.
Continue ReadingSupply Chain Agility: A Challenge or an Opportunity? (Part 1 of 3)
There is an old expression: “May you live in interesting times.” The origin is unclear, as some attribute it to a centuries-old Chinese curse.
Continue ReadingHealthcare Supply Chains Complying with the Drug Quality and Security Act
The healthcare industry is racing to meet the November 2023 deadline set forth by The Drug Quality and Security Act (DQSA). The purpose of the DQSA is to build an electronic system to track and trace certain prescription drugs as they are distributed in the United States.
Continue ReadingCritical Options to Make Your Company More Flexible, Scalable and Agile
From the time I joined Sedlak several decades ago until today, we have recommended to clients solutions that provided the potential to be scalable and flexible. Accomplishing this in the past meant that where possible, especially in an expansion or new build, we would allow additional space for expansion of areas.
Continue ReadingPeak Season is right around the corner. Is your team ready?
If last year taught us anything, it’s that it’s never too early to plan. In fact, the best time to start planning is immediately following peak season – but now is better than never!
Continue ReadingAutomation with Inther Group in the Face of Labor Shortages
The Sedlak Team was fortunate enough to have Inther Group, an international system integration firm, host a Lunch and Learn session for us recently. Inther Group automates internal logistic processes by integrating manual solutions and mechanized systems.
Continue ReadingWhy Internal Resources Need More Help Than You Might Think
Why does a company seek help from an outside consulting firm? Usually the reasons are simple: they lack the manpower, bandwidth and/or skillset needed to complete their projects.
Continue ReadingOptimizing Supply Chains in Response to Ocean Freight Shipping Challenges
Over a quarter of the way into 2021, supply chains are still reeling from the effects of the COVID-19 pandemic. Companies have been reviewing ways to sustain and even improve business moving forward, especially when it comes to transportation.
Continue ReadingThe Evolving State of AGVs
The Automated Guided Vehicle (AGV) market is evolving at a rapid pace. As recently as three years ago, AGVs were primarily relegated to simple horizontal moves of pallets over longer distances, either transporting one or two pallets at a time using a system such as Seegrid or lined up in trains with a tugger pulling several carts of pallets.
Continue Reading2020: A Year to Remember for Logistics Professionals
In my 40+ years of providing logistics and distribution consulting services to clients, no other year has had a greater impact on the profession than 2020. For an industry already dealing with labor shortages, the need to embrace higher levels of automation became critical as order characteristics morphed to significantly smaller direct-to-consumer characteristics.
Continue ReadingWhy Hyper-local is the Future of Fulfillment
Micro-fulfillment has taken off in the past several years, but what about hyper-local fulfillment? Can your company take advantage of this growing trend?
Continue ReadingTaking Control of Your Operations During COVID
Have you reviewed the priorities of your operations during the COVID-19 epidemic? How has COVID-19 opened up time and opportunity for your company to tackle new activities?
Continue ReadingHow to Handle the New Normal in 3 Steps
So far, Sedlak and several of our clients have been experiencing the effects of the “New Normal” on the supply chain and taking steps to mitigate them. Three of these migitation steps include (1) strategy, (2) project activities & communications, and (3) operational design.
Continue ReadingModular Construction and Its Impact on Supply Chain
We’re all seeing how modular construction is becoming more and more of a requirement on many job sites. It makes sense: contractors pre-assemble components in a controlled manufacturing environment, which improves quality, and delivers them to the project ready-to-install.
Continue ReadingFinding the Right TMS for an International Shipper
When selecting a TMS, it is paramount to partner with one that best fits your needs to realize that ROI. Organizations with a low domestic freight spend but high international shipment volume need a Tier 1 TMS.
Continue ReadingHow to Cut Costs and Conflict When Choosing a TMS
TMS is hot right now, and it has shippers and 3PLs excited. Competition among top TMS providers and new entrants have yielded better tools and reduced product costs.
Continue ReadingTime is Running Out to Deploy Automated Pop-up Micro-fulfillment Facilities
If your business is preparing for the common holiday peak, time is running out to take the proper measures. Using a few familiar concepts, we can help quickly deploy a flexible, effective solution that will assist a number of our clients as they face so many unknowns in 2021.
Continue ReadingMaking the Leap to Manhattan Active WM with Sedlak
Sedlak Supply Chain Consultants has paved the way for enterprise-level companies to select, implement and execute Manhattan Associates’ new cloud-native WMS with Manhattan Active Warehouse Management.
Continue ReadingHave You Adapted to the “New Normal”?
The world has been turned upside down due to the COVID-19 pandemic. The global economy is in a tailspin and the prediction of a quick recovery is becoming less likely as mention of a “new normal” begins to dominate the headlines.
Continue ReadingHow to implement an LMS in under 120 days
If, like many of your counterparts, you are faced with the challenges of finding and keeping a motivated labor force, all while reducing costs and meeting executive expectations for 2020, time is not your friend.
These labor challenges have been exacerbated further by the COVID-19 outbreak, making it critical for your business to take action and prepare for the demand and labor challenges that will come once the crisis is behind us.
Continue ReadingTransform Your Network into an Agile Supply Chain
The modern supply chain has never experienced a crisis quite like the COVID-19 pandemic. It’s rare to see countrywide company shutdowns.
Continue ReadingLMS Implementation Costs and Schedule
You’ve just been tasked by your COO with the responsibility of selecting and implementing a labor management system as part of your supply chain operation. What do you do?
Continue ReadingUtilizing Change Management to Ensure Labor Management Success
If the tight labor market and increased demand for improved customer service are a constant challenge, a labor management system (LMS) can help to mitigate these issues. A successful LMS can be secured through a properly designed and integrated change management program.
Continue ReadingThe Crucial Benefits of Labor Management Systems (LMS)
Ever-tightening labor markets, coupled with increasing customer delivery expectations, are forcing operations managers across industries to do more with less. A great tool to assist the management of resources is a competent Labor Management System (LMS) software package.
Continue ReadingInsight into Optimizing Labor
With the seasonal peak in the rearview mirror, it is time to reflect on the season past and look forward to the year ahead with a clear goal of addressing the challenges from 2019 and developing a strategic plan for 2020 and beyond.
Continue ReadingModular Construction: How Does it Impact the Supply Chain?
In modular construction, trusses are assembled offsite and delivered to the property, erected as a whole by a crane, and fastened in place by a carpenter. This practice has become more and more popular, and now companies are adopting it for their manufacturing and distribution facilities.
Continue ReadingCrucial Preparations for Optimizing Current- and Future-State Peak Season Performance
Now is the critical time to perform supply chain execution evaluations for peak season planning. Listed below are some recommendations to consider regarding a few key factors influencing 2019 end-of-year performance.
Continue ReadingSedlak Supply Chain Consultants Featured in Retail Merchandiser and in Supply Chain World
After celebrating 60-years in business, Sedlak Supply Chain Consultants was recently featured in Retail Merchandiser and Supply Chain World. With a focus on Sedlak’s healthcare and retail clients, the article highlights challenges common across industries, and provides examples of Sedlak’s ability to bring order from chaos Excerpt: “We take a long view of our client […]
Continue ReadingPatrick Sedlak quoted in Modern Materials Handling
Patrick Sedlak, Principal at Sedlak Supply Chain Consultants, was quoted in a recent article on the Modern Materials Handling website. In an article titled “Single Source of Truth with Computerized Maintenance Management Systems (CMMS)” by Roberto Michel, Sedlak was asked for his thoughts on whether DCs should use one CMMS or two.
Continue ReadingFinding Savings While Improving Service in Healthcare
Sedlak Supply Chain Consultants conducted a Routing Optimization Analysis for MetroHealth in Cleveland, OH to optimize the efficiency of its Integrated Delivery Network (IDN).
Continue ReadingPart III of Fred Crans’ Supply Chain Disruption Series Published in Healthcare Purchasing News
What does Paul Simon have to do with the healthcare supply chain? Find out by reading Part III of Fred Crans’ series on supply chain disruption, published in the September 2018 edition of Healthcare Purchasing News.
Continue ReadingSedlak Celebrates 60 Years Serving Over 1,200 Clients
Cleveland, OH—Sedlak Supply Chain Consultants, an independent distribution and logistics consulting firm, is celebrating its 60th anniversary this month. Sedlak has helped more than 1,200 clients worldwide with over 2,500 projects since it was founded, providing services including network strategy, facility planning and design, operations improvement, transportation planning, inventory optimization, change management and IT systems […]
Continue ReadingSedlak Announces Kara Ashby as its New President
Sedlak Supply Chain Consultants, a distribution and logistics consulting firm based in Cleveland, is pleased to announce Kara Ashby as its new president. Just the fourth president in the history of the company, Ashby is the first woman to take the reins at Sedlak.
Continue ReadingLearning New Things in Healthcare Supply Chain
A year and a half ago I came to Sedlak as a 40+ year veteran of the healthcare supply chain. I thought my charge was to teach these Industrial Engineers about healthcare.
Continue ReadingSedlak Congratulates Mills Fleet Farm on Opening of New Distribution Center
Just one year after breaking ground, Mills Fleet Farm opened its new 1.1 million sq.
Continue ReadingWhy It May Be Time to Consider a New WMS
A Warehouse Management System (WMS) is considered an essential component of the modern supply chain. Learn: 1) Why you should consider a WMS; and 2) What you should know if you are considering one.
Continue ReadingAutomation to AI – Moving at the Speed of Supply Chain Technology
Technological advances supporting the supply chain are accelerating the pace of change. Don’t assume your business is too small or too set in its ways to benefit.
Continue ReadingDon’t Neglect Basic Healthcare Supply Chain Functions
As health care organizations focus on improving operations in order to become more efficacious and cost-effective in rendering care, they often focus on big picture initiatives. In doing so, they can overlook opportunities in more mundane places.
Continue ReadingRFID Advantages Over Barcodes in the Distribution Center
Radio Frequency Identification (RFID) offers significant supply chain benefits not provided by traditional barcode labels. Do the advantages make this technology right for your distribution operation?
Continue ReadingWhat Healthcare Can Learn from Retail, Grocery – Interview with Healthcare Purchasing News
Healthcare supply chain management is a constant balance of cost, quality, and outcomes. The storage and movement of medications, equipment, and supplies is a substantial link in that chain.
Continue ReadingSedlak Examines Retail Trends, Innovations, and Opportunities in MHI Solutions
The growth of e-commerce, consumer price sensitivity, online “convenience” shopping, the Amazon effect, and the premise of returns are all driving fundamental changes in the retail industry. Supply chains and distribution best practices are rapidly evolving to support super flexible customer fulfillment and compete with the big dogs.
Continue ReadingTim Harlan Named 2017 Associate of the Year
Sedlak congratulates Director of Client Services Tim Harlan on being named Associate of the Year for 2017. Tim has been with Sedlak for 12 years and has over 34 years of distribution and consulting experience with demonstrated success in process development and operations management.
Continue ReadingDennis Heppner Discusses Last Mile Delivery Collaboration with MHI Solutions
New entrants in the last mile market in the U.S.
Continue ReadingGreetings of the Season from Your Friends at Sedlak!
Best wishes for a joyous Christmas and a happy and prosperous New Year!
Continue ReadingRickenbacker International Airport Leads Freight Innovation in Air Cargo
Sedlak is proud to see Rickenbacker International Airport (LCK) and the Columbus Regional Airport Authority featured as an air cargo innovator in this month’s issue of Inbound Logistics. In October, Sedlak partnered with CSCMP to host a tour of the Rickenbacker Air Cargo Terminal which featured a flight landing by Emirates.
Continue ReadingJeff Mueller talks about Amazon Key In-Home Delivery Offering with Internet Retailer
[From Internet Retailer] Amazon.com Inc.
Continue ReadingThe Limited Returns as an E-retailer
Women's apparel brand The Limited relaunched itself last week as an on-line only store, nearly a year after it filed for Chapter 11 bankruptcy protection and liquidated its retail assets. According to an article on Internet Retailer, retail experts say The Limited's established customer base gives the brand a good chance at success as an e-commerce […]
Continue ReadingPop-up Stores? Now Pop-up Distribution Centers
Pop-up stores in malls, airports, and other places have been around forever. Now the concept is gravitating to pop-up distribution centers, where there may be some very real benefits for retailers facing labor shortages in their current distribution areas.
Continue ReadingShipping Costs: Putting Freight Fuel Efficiency into Context
Long-haul tractor trailers get a bad rap for averaging 7 miles per gallon of diesel, but in context they are more fuel efficient than a Ford F250 pickup, and various innovations are improving fuel economy.
Continue ReadingConsolidated Services Centers – Before You Take the Plunge (Part 2 of 2)
A Consolidated Services Center (CSC) can create savings for a healthcare organization if executed well. However, the decision should be well thought out and justified with extensive research and financial modeling as it represents a substantial investment with ongoing requirements.
Continue ReadingConsolidated Services Centers – Before You Take the Plunge (Part 1 of 2)
A Consolidated Services Center (CSC) can create savings for a healthcare organization if executed well. However, the decision should be well thought out and justified with extensive research and financial modeling as it represents a substantial investment with ongoing requirements.
Continue Reading‘Tis the Season to Battle for Warehouse Labor
Labor is typically among the top operational costs in a distribution center, and the exploding ecommerce demand for warehouse workers is creating a shortage of laborers that is driving up the costs of recruiting, hiring and training for the companies who need them.
Continue ReadingSedlak Receives Business Longevity Award by Smart Business
Sedlak was recently named a recipient of the 2017 Business Longevity Award by Smart Business, which recognized Northeast Ohio businesses that have achieved business milestones of 50, 75 or 100 years. Presented with the award plaque at a luncheon at the Western Reserve Historical Society in August, Sedlak was recognized for being a company built on […]
Continue ReadingSedlak Congratulates DSW for Topping Total Retail’s Top 100 Omni-Channel Retailers Ranking
Sedlak congratulates branded footwear and accessories retailer DSW for earning a top spot in Total Retail’s inaugural ranking of 100 publicly traded retailers based on their omnichannel capabilities. [From Total Retail] DSW received the top possible score (100) across the seven criteria — buy online, pick up in-store; search in-store products online; shared cart; loyalty points earned/redeemed across channels; […]
Continue ReadingNew Mills Fleet Farm Distribution Center in Chippewa Falls, WI
Mills Fleet Farm, which sells everything from hunting gear and lawn equipment to hardware and automotive supplies to clothing and branded foods, plans to build a 1.1 million square-foot distribution center in Chippewa Falls, Wisconsin.
Continue ReadingQ&A: Labor Management…Works!
Every company seeks maximum return on investment, and labor is one of the most expensive assets in a distribution center. A labor management system (LMS) is an integral part of realizing the maximum benefit of both the people and technologies in a distribution operation.
Continue ReadingSedlak to Lead Advanced 3PL Management Workshop at Pharma Supply Chain Conference
The Supply Chain Operations & Compliance in Pharma is a 3-day industry-led meeting which: Offers a deep dive into current operational challenges and solutions in your pharma supply chain Assesses global regulatory landscapes and provides you with the insight to navigate Helps you understand and define a risk management framework, and ensures your 3PLs are […]
Continue ReadingSedlak Congratulates Principal Jeff Graves on 45th Anniversary with the Firm
Principal Jeffrey B. Graves is celebrating 45 years of a very distinguished career with Sedlak, a company which he has devotedly built into a premier independent supply chain consulting firm specializing in distribution and logistics.
Continue ReadingSedlak Celebrates 20-Year Anniversary as a Manhattan Associates Partner
Sedlak celebrates its 20th year as a partner of Manhattan Associates. The company was selected as Manhattan’s first Certified Alliance Partner in 1997 and has maintained a strategic partner status, driving successful collaboration with joint customers.
Continue ReadingSedlak Named Best Consulting Partner by Manhattan Associates
Sedlak was recently named to Manhattan Associates’ distinguished Partner Performance Club in recognition of significant contributions over the past year to customer engagements. As a strategic partner, Sedlak helps drive successful collaboration with joint customers.
Continue Reading5 Reasons to Consider Hydrogen Fuel Cells over Batteries for Mobile Equipment
If considering adding new mobile equipment to an operation or replacing a fleet, hydrogen fuel cells are certainly worth consideration. Here are five reasons why.
Continue ReadingSedlak Congratulates Manager Les Grimes on His Retirement After 18 Years of Outstanding Servce
Congratulations to Manager Les Grimes on his retirement after 18 years of outstanding service with the firm. When Les joined Sedlak in 1999, he brought over 20 years of operations experience in the consumer products industry to our knowledge base.
Continue ReadingTransportation Tipping Points in Healthcare Logistics
Transportation costs are defined by volume – aggregating into larger volumes will result in lower costs. As healthcare companies grow and expand their business, it’s important for them to evaluate where their volumes are.
Continue ReadingJeff Mueller Talks Warehouse E-commerce Best Practices for 2017 in MMH Magazine
As manufacturers, distributors and retailers all strive to run efficient operations that not only meet the demands of their traditional businesses, but also support their e-commerce operations, the race is on to develop efficient, optimized warehouses and DCs to support these multifaceted operations. Driven by the sheer volume of products and services being sold online—a […]
Continue ReadingCloud Logistics Wins Industry Awards
Sedlak congratulates Cloud Logistics, an innovator and provider of next-generation transportation management systems (TMS), for recently being named the recipient of two industry awards. The company was named to the Food Logistics 100+ Top Food & Beverage Software and Technology Providers list, a resource guide of software and technology providers whose products and services are […]
Continue ReadingPatrick Sedlak and Jeff Mueller Stress Agility for Omni-channel Fulfillment in MHI Solutions Magazine
Flexibiliy and agility are essential for retailers striving to provide a seamless shopping experience across all channels. Principal Patrick Sedlak and Vice President Jeff Mueller are quoted in MHI Solutions magazine on how the distribution center environment is changing – streamlining processes and adding automation – to keep up with customer demand and address peak season challenges.
Continue ReadingSedlak Accounting Manager Denise Bybyk Retires After 22 Years of Service
Sedlak bid a fond farewell and heartfelt congratulations to Accounting Manager Denise Bybyk, who has retired after 22 years of exemplary service with the firm. “She has always kept us on course,” said Principal Jeff Graves at her retirement luncheon, “and is one of the most dedicated and loyal associates I have known.
Continue ReadingSeason’s Greetings from Sedlak!
Wishing you a Happy Holiday season and a Remarkable New Year! [Click image to play.
Continue ReadingPurchased Services Program Offers Savings for Healthcare Supply Chains
Fred Crans recently presented a roadmap for managing purchased services at the Strategic Marketplace Initiative Fall Forum.
Continue ReadingSedlak Applauds Patagonia for $10M Black Friday Donation
We certainly weren't surprised when we saw the headline that Patagonia was donating 100% of its Black Friday sales this year to grassroots environmental groups it supports. “We worked for Patagonia for many years, and it completely changed my life,” says Sedlak vice president Jeff Mueller.
Continue ReadingThe Supply Chain at the End of the Earth (3 of 3)
This three-part series explains Sedlak’s work in support of the Antarctic Infrastructure Modernization for Science (AIMS) project of the U.S.
Continue ReadingThe Supply Chain at the End of the Earth (2 of 3)
This three-part series explains Sedlak’s work in support of the Antarctic Infrastructure Modernization for Science (AIMS) project of the U.S.
Continue ReadingThe Supply Chain at the End of the Earth (1 of 3)
This three-part series explains Sedlak’s work in support of the Antarctic Infrastructure Modernization for Science (AIMS) project of the U.S.
Continue ReadingFrom Our Inbox: Delivering Remarkable Experience to Fresh Mark
Sedlak’s expertise in material flow and operational process improvement benefits manufacturers as well as distributors. We were pleased to receive this testimonial for our recent work with Fresh Mark, a producer of quality meat products.
Continue ReadingManaging Change – IDN Summit 2016
A need for industry-wide change management competencies to counter the ever-shifting demands in healthcare was a key focal point at the Fall IDN Summit held September 19-21 in Phoenix.
Continue ReadingLogiPharma Roundtable: Security, Labor, Efficiency – Material Handling Automation in Healthcare
While decisions around distribution operations and the levels of automation in those environments are varied in the healthcare industry, a key driver for some companies is overall organizational strategy rather than a specific focus on security, labor, cost or efficiency. Regardless, there is awareness in the pharmaceutical industry that warehousing is on the brink of […]
Continue ReadingManaging Healthcare Logistics Spend – A Vital Corporate Function
For the healthcare industry, the delivery of products – from manufacturers to distributors to providers to patients – is often an isolated entity in the supply chain. Dennis Heppner presents reasons why a holistic and proactive approach to healthcare logistics management is critical to maintain performance and profitability.
Continue ReadingUnder Armour Announces Plan To Open E-Commerce Distribution House In Maryland
Under Armour plans to open a 1.3 million square-foot distribution and warehouse facility at Sparrows Point in Baltimore County, as part of the Tradepoint Atlantic redevelopment project.
Continue ReadingSecurity, Labor, Cost, Efficiency: Material Handling Automation in Healthcare
A Sedlak roundtable at LogiPharma addressed drivers behind distribution operations and automation in the pharmaceutical industry.
Continue ReadingSedlak Congratulates Kara Ashby on 20th Anniversary
Congratulations to Kara Ashby, Director of Client Services, who celebrates 20 years with Sedlak today. Her major area of expertise is in warehouse management systems (WMS) design, implementation and support.
Continue ReadingPatrick Sedlak Quoted in Huffington Post on Self-Driving Transportation
Patrick Sedlak is quoted in an article by Thomas Kaufmann on movers and shakers in self-driving technology. From preventing collisions to cloud-to-car mapping systems to artificial intelligence, innovations are on track to upend over one hundred years of human driving tradition.
Continue ReadingSedlak Featured in Business View Magazine
Whether it’s retail, consumer packaged goods, third party logistics, wholesale distribution, or healthcare management, Sedlak Management Consultants continues to “Deliver Remarkable Experience” to its clients. “We're a highly effective and highly cilent-oriented firm,” said Sedlak President Will O'Brien.
Continue ReadingPatrick Sedlak, Kara Ashby will be Session Panelists at CSCMP’s Annual Conference
CSCMP’s 2016 Annual Conference is an event like none other, presenting you with unlimited opportunities to learn from industry experts, hear dynamic speakers, and network with the most influential supply chain leaders in the world. You’ll also connect to the latest supply chain management knowledge, research, and industry developments.
Continue ReadingFrom Our Inbox: Delivering Remarkable Experience to VF Corporation
Sedlak has built a long-standing relationship with VF Corporation, a leader in branded lifestyle apparel and footwear, that includes over 25 strategic and facility planning efforts across multiple business units since 1993. So we were especially pleased to find this recent email in our inbox: “So pleased to see ‘Delivering Remarkable Experience’ as your new tag […]
Continue ReadingFound Money: How to Clean Up Preference Cards to Cut Inventory Spend (3 of 3)
This three-part series concludes by identifying how hospitals can sustain the benefits and savings of preference card management.
Continue ReadingFound Money: How to Clean Up Preference Cards to Cut Inventory Spend (2 of 3)
Part two of this three-part series outlines actions hospitals should take identify their losses and capture potential savings by cleaning up existing preference cards.
Continue ReadingFound Money: How to Clean Up Preference Cards to Cut Inventory Spend (1 of 3)
This three-part series examines the potential for hospitals to capture inventory savings through more efficient management of physician preference cards.
Continue ReadingLou Cerny is Co-Chairing Technology Track at CSCMP Annual Conference
CSCMP’s 2016 Annual Conference is an event like none other, presenting you with unlimited opportunities to learn from industry experts, hear dynamic speakers, and network with the most influential supply chain leaders in the world. You’ll also connect to the latest supply chain management knowledge, research, and industry developments.
Continue ReadingPatrick Sedlak to Lead MHE Roundtable at LogiPharma
With the fabric of healthcare distribution in flux, the challenge for pharmaceutical distribution executives is focused on how to leverage distribution infrastructure to reduce costs, but maintain the flexibility needed to mitigate risk across the supply chain. Principal Patrick Sedlak will serve as the subject matter expert for a roundtable discussion on “Automation Innovations in […]
Continue ReadingTrusted Solutions – Sedlak Profiled in Retail Merchandiser Magazine
“We’ve got a very rich history in retail,” Principal Patrick Sedlak said in a recent interview featured in Retail Merchandiser Magazine. For nearly 60 years Sedlak has been helping retailers develop and maintain the optimal strategies, facilities, operations and systems to meet the growing challenges of multi-channel and omni-channel fulfillment.
Continue ReadingHow a Formal Network Design Capability Can Help Medical Products Manufacturers Thrive (4 of 4)
The conclusion of this four-part series offers advice on how companies can decide on the best approach to developing a network capability.
Continue ReadingSedlak Celebrates 58 Years of Delivering Remarkable Experience
Celebrating 58 years of Delivering Remarkable Experience! Sedlak celebrates its “Beddian” birthday today — a term coined by New York firefighter Bobby Beddia to mean the year when your age matches your birth year.
Continue ReadingHow a Formal Network Design Capability Can Help Medical Products Manufacturers Thrive (3 of 4)
Part three of this four-part series explores options available to medical products manufacturers to achieve their own robust network design capability.
Continue ReadingSupplying Expertise – Sedlak Featured in Supply Chain World Magazine
The increasing complexities and challenges of the retail supply chain resulting from consumer demands for an “anytime, anywhere” shopping experience can be overwhelming for many retailers. For nearly 60 years, retailers of all sizes and types have trusted Sedlak to find the right answers for their distribution and logistics needs.
Continue ReadingDave DuBose Links Retail S&OP to a Smoother Holiday Season in Multichannel Merchant
In the July issue of Multichannel Merchant, Director of Supply Chain Solutions Dave DuBose discusses how the S&OP process, historically a planning tool more commonly used by manufacturers, can help retailers collaborate internally to anticipate and prepare for shifts in demand. “With the 2016 holiday season only five months away, it’s a good time for retailers […]
Continue ReadingDemystifying Freight Spend: How Shippers Can Get a Better Handle on Their Carriers (2 of 2)
Part two of this two-part series examines how shippers can navigate their carrier relationships and more effectively control freight spend, including rate hikes.
Continue ReadingSedlak Congratulates VP Jeff Mueller on 25 Years
Congratulations to Jeff Mueller on his 25th anniversary with Sedlak! Jeff serves as vice president with major responsibility for assessing clients’ distribution and logistics challenges and leading information technology efforts.
Continue ReadingHow a Formal Network Design Capability Can Help Medical Products Manufacturers Thrive (2 of 4)
Part two of this four-part series details the essential activities of a network analysis and design capability.
Continue ReadingDemystifying Freight Spend: How Shippers Can Get a Better Handle on Their Carriers (1 of 2)
This two-part series details the factors that affect the price shippers pay carriers to move freight and how shippers can more effectively control freight spend. Part one of this series details the factors and complexities affecting the price shippers pay carriers to move freight.
Continue ReadingHow a Formal Network Design Capability Can Help Medical Products Manufacturers Thrive (1 of 4)
This four-part series explores why medical products providers need a formal network design capability to remain competitive and how to achieve it.
Continue ReadingSedlak Congratulates Associates on Recent Certifications
Sedlak congratulates Senior Consultant Davon Soltis for successfully completing certification in JDA’s TMS Bootcamp, and Director of Client Services Brian Neale and Consultant Lisa Betts for completing certification in Change Guide’s Change Management program. Soltis achieved a near perfect score on the TMS final exam.
Continue ReadingChange Management: A Critical Component of Project Success
Sedlak consultant Lisa Betts recently completed certification in change management with Change Guides. She provides some of her insights from the course in Change Guides' spring newsletter and in today's post.
Continue ReadingStructuring the Medtech Supply Chain for Growth
Posted on April 12, 2016, at Device Talk, a blog by the Medical Device and Diagnostic Industry (MDDI), Steve Simco discusses how medical device manufacturers can build an internal capability for network design to provide a competitive advantage in the face of rising costs and changing dynamics in the healthcare industry. “It’s likely that disruptive […]
Continue ReadingSedlak Assists British Columbia LDB in Development of New Distribution Center
Sedlak is assisting the British Columbia Liquor Distribution Branch (LDB) with the design and deployment of a new warehouse and distribution center in the Lower Mainland, BC, Canada. The new design incorporates upgraded warehouse systems to create efficiencies and improve service levels at lower operating costs.
Continue ReadingSedlak Recognized as a Specialist in Supply Chain Strategy & Operations Consulting
Sedlak, a long-standing supply chain and distribution logistics advisory firm, has been recognized by Gartner as a specialized supply chain and operations authority in Gartner’s inaugural “Market Guide for Supply Chain Strategy & Operations Consulting.” The guide is designed to help CSCOs and functional supply chain leaders find appropriate industry consultants for their supply chain […]
Continue ReadingStretching the Boundaries of Security: MHE in Healthcare
In today’s healthcare world, especially in pharmaceuticals, security often reigns paramount in decision-making processes. Interestingly, in one of the most mundane of operational processes in the supply chain, pallet “wrapping”, options exist that have dramatic effects on the “cost of security” trade off.
Continue ReadingLou Cerny Quoted in Supply Chain Function Article
Online shopping is transforming the way manufacturers of consumer products do business and think about their supply chain. Supply chains now touch every corner of the business and systems integration provides real-time data on everything from inventory and sales to consumer behaviors and labor management.
Continue ReadingPatrick Sedlak and Will O’Brien Quoted in MHI Solutions Magazine Article
The rise of omnichannel, as well as increasingly demanding fulfillment requirements, present technological challenges to the retail apparel industry. A retailer’s ability to create a seamless experience for shoppers, regardless of how they choose to shop – in store, online, mobile – is dependent on automation with precision to track inventory, speed to fulfill orders, […]
Continue ReadingLessons Learned from Sedlak’s Network Design Project in SC Digest
David Zuern, VP of Logistics from Benjamin Moore Paints discussed his network design project (by Sedlak) at the CSCMP annual conference in San Diego. The paint manufacturer that differentiates itself with very high service standards learned 5 critical things during its network study with Sedlak.
Continue ReadingAscena Retail Group Opens Ecommerce Fulfillment Center
Sedlak celebrated with client Ascena Retail Group, Inc., at the formal opening of its world-class ecommerce fulfillment center in Greencastle, Indiana on May 14.
Continue ReadingThe Perils and Promise of Omni-Channel Returns
As online sales grow, so does the volume of returns grow…so much so that the total volume of returns is now greater that the total volume of e-commerce orders! How retailers deal with returns has a significant impact on their customer experience and retention, as well as their overall margin and economics.
Continue ReadingRaising the Roof Making All the Difference in Warehouses
The Wall Street Journal investigates a growing trend for higher ceilings in warehousing and fulfillment facilities. Building vertically rather than horizontally allows omni-channel retailers to use 3-level pick modules and taller storage rack configurations for efficient fulfillment of e-commerce orders while minimizing the land requirement.
Continue ReadingThe Business Case Behind LEED Certification
Should companies undertake the effort and expense involved in LEED certification or simple adopt eco-friendly practices? Lou Cerny makes the point that while LEED is the right ethical direction for companies, there needs to be a strong set of business reasons to support the certification process.
Continue ReadingPharma Supply Chain: Changing Dynamics
“With the fabric of healthcare distribution in flux, the challenge for pharmaceutical distribution executives is focused on how to leverage distribution infrastructure to reduce costs, but maintain the flexibility needed to mitigate risk across the supply chain. “Pharmaceutical distribution, like with any other industry, has its peculiarities that require specialized applications to keep products moving […]
Continue ReadingLou Cerny Discusses Labor Management Programs with SupplyChainBrain
A company’s most important assets are its employees, and acquiring and retaining top performing associates is one of the largest factors in cost savings. Companies are finally beginning to recognize the benefits of labor management systems (LMS) in their distribution centers.
Continue ReadingCollaboration in the Healthcare Value Chain: Lessons from the Grocery Industry
While it’s clear there is concern and frustration regarding the uncertainties created by the political scene and rapidly changing regulatory environments, energy regarding change is apparent everywhere in healthcare distribution.
Continue ReadingDistribution Strategy and the Changing Dynamics of the Pharmaceutical Supply Chain (Pt. 4)
With the industry-wide changes occurring within the healthcare supply chain, many pharmaceutical manufacturers and distributors are reassessing their distribution network models. In part four of this series, we outline the challenges and primary functions of distribution network strategy development.
Continue ReadingDistribution Strategy and the Changing Dynamics of the Pharmaceutical Supply Chain (Pt. 3)
In part three of this series we discuss why supply chain optimization and management is rising to the top of cost reduction strategies among C-level executives in healthcare.
Continue ReadingDistribution Strategy and the Changing Dynamics of the Pharmaceutical Supply Chain (Pt. 2)
Already a challenging distribution environment, a combination of demographic trends, healthcare legislation, new drug introductions and other factors are elevating the visibility and importance of healthcare logistics and distribution activities. In part two of this series, we focus on the market shifts impacting pharmaceutical distribution.
Continue ReadingUnder Armour Breaks Ground on 3rd Distribution House in Mt. Joliet, TN
Sedlak congratulates Under Armour on the recent groundbreaking for its new Distribution House in Mt. Joliet, TN, a suburb of Nashville.
Continue ReadingLou Cerny Discusses Sustainability and Risk on CSCMP Panel
Lou Cerny participated in a panel discussion on Sustainability and Risk Management at the CSCMP Annual Conference in San Antonio, TX. The session focused on how leaders are cutting costs by weighing environmental compliance and corporate responsibility when making facility design, operations and procurement decisions.
Continue ReadingDistribution Strategy and the Changing Dynamics of the Pharmaceutical Supply Chain (Part 1)
With the fabric of healthcare distribution in flux, the challenge for pharmaceutical distribution executives is how to leverage distribution infrastructure to reduce costs, but maintain the flexibility needed to mitigate risk across the supply chain. In this five-part series we discuss dynamics in pharmaceutical distribution, market shifts impacting pharmaceutical distribution, optimizing distribution processes, key components of a distribution network assessment and leveraging a broader perspective.
Continue ReadingThe Nemesis of Supply Chain Data: From Obscure and Disorderly to Visible and Aligned
Data is a four letter word - especially to supply chain practitioners across most industries. Data is that unmentionable thing we all love to hate but hate to ignore.
Continue ReadingStreamline Your WMS Start-Up (Pt. 4)
A tough WMS start-up does not just happen in the two weeks prior to go-live. These scenarios are caused by inadequate pre-planning from the very beginning, and poor project management.
Continue ReadingStreamline Your WMS Start-Up (Pt. 3)
Some WMS installations have gotten so stalled that they were eventually shelved and abandoned. In part three of this series, we discuss the importance of planning.
Continue ReadingStreamline Your WMS Start-Up (Pt. 2)
In part two of this series, we explore the risks affecting WMS implementation. Despite the importance of WMS, approximately 30 percent of installs fall behind schedule and fail to be ready for operation at go-live.
Continue ReadingStreamline Your WMS Start-Up (Pt. 1)
Despite their importance, successful implementations of warehouse management systems (WMS) are seldom fully realized in highly automated distribution centers. Topics covered in this four-part series include, warehouse management system goals, risk affecting WMS implementation, planning and implementation insurance.
Continue ReadingSupply Chain IT in Healthcare: Staying in the Eye of the Storm
In highly changing environments like healthcare, the organizations that are most successful in the long term are the ones that learn how to adapt and evolve - especially in the areas of processes and systems.
Continue ReadingSedlak Sets Up DSW for Future Growth with Sortation System
DSW is featured in Modern Materials Handling for its new cross-belt sortation system. Sedlak’s system design, procurement and project management services helped DSW increase store replenishment six-fold and double productivity at its Columbus DC.
Continue ReadingIs Your Healthcare Supply Chain Network Ready to Adapt?
It seems evident that everything in the healthcare industry is changing – customer profiles, number and size of suppliers, product characteristics, service requirements, transportation needs, etc. As a result, the ability to quickly and efficiently perform a supply chain network “optimization” analysis is becoming increasingly relevant.
Continue ReadingIndependent Logistics Consultants vs. Systems Integrators (Pt. 5)
In part five of this series, we explore change management and how it is a critical link in ensuring the smooth transition of new and upgraded DCs into functionally efficient live operations.
Continue ReadingIndependent Logistics Consultants vs. Systems Integrators (Pt. 4)
Part four of this series focuses on designing a DC from an automation focus delineates a significant difference in how systems integrators and independent logistics consultants approach distribution solutions.
Continue ReadingIndependent Logistics Consultants vs. Systems Integrators (Pt. 3)
In part three of this series, we discuss how the methodology of independent logistics consultants and a systems integrator may differ when it comes to objectivity in system design.
Continue ReadingIndependent Logistics Consultants vs. Systems Integrators (Pt. 2)
Part two of this series examines some of the important questions that drive facility design decisions and how an independent logistics consultant like Sedlak Management Consultants, Inc., brings a depth and breadth of knowledge to guide the best-fit and most cost-efficient solutions for the client.
Continue ReadingIndependent Logistics Consultants vs. Systems Integrators (Pt. 1)
Although systems integrators position themselves as single-source suppliers of distribution solutions, the increasingly complex nature of supply chains demands the broader scope of experience and unbiased methodologies of the independent logistics consultant. This five-part series addresses key questions and essential factors that make the choice of partner clear.
Continue ReadingSupply Chain’s Role in Driving Growth
At Sedlak Management Consultants we believe that a well-planned and well executed supply chain should be a key asset in driving the overall growth and success of a company. Cost and efficiency are important, but providing services and enabling sales by being in stock and able to get product into customer’s hands when and where they want it is a key part of supporting sales growth.
Continue ReadingQuaint is the Pioneer’s Curse: Technology in Retrospect
Only ten years ago, the Internet and distribution were considered a relatively new partnering. MH&L columnist Tom Andel reflects on how rapidly times have changed as he remembers two material handling pioneers, Joseph Sedlak and Bernie Krill.
Continue ReadingSedlak Earns Spot on Supply Chain Brain’s Top 100 Supply Chain Partners
Sedlak was one of hundreds of companies from every aspect of supply chain management nominated during a six-month online poll in which supply chain professionals were asked to nominate vendors and service providers whose solutions have made a significant impact on their company’s efficiency, customer service and overall supply chain performance. Now in its 11th […]
Continue ReadingVF Corporation Reopens Wrangler DC 2 Years After Deadly Tornado
“This plant doesn’t just mean a job, it means a way of life,” said Alabama Gov. Robert Bentley.
Continue ReadingRetail : DC at the Center of it all
Jeff Mueller is featured in the April issue of Modern Materials Handling. Read the complete article here.
Continue ReadingStreamline Your WMS Start-Up
“Despite their importance, successful implementation of warehouse management systems (WMS) is seldom fully realized in highly automated distribution centers. This is evident in the many WMS implementations of new and upgraded distribution centers that are not adequately functional or fully utilized by staff—in some cases even years after they were targeted to be completed.
Continue ReadingMaximizing Productivity in E-Commerce 3PLs
Jeffrey Graves contributed an article in the December 2012 issue of 3PL Americas. “As e-commerce continues its rapid growth into virtually every market sector, retailers are anxious to expand their presence online to capture this market share.
Continue ReadingWhat You Should Know About Goods-to-Person Fulfillment
The recent introduction of several goods-to-person order fulfillment systems calls for a thorough understanding of their potential fit into any logistics operation. Read Jeff Graves’ recent article about Goods-to-Person Fulfillment here.
Continue ReadingWrangler Distribution Center Breaks Ground on New Facility Following April 2011 Tornadoes
On April 24 in Hackleburg, Alabama groundbreaking ceremonies were held on the site where VF Corporation’s plant was destroyed when a tornado ripped through the town on April 27, 2011. The VF Wrangler plant is Hackleburg’s largest employer.
Continue ReadingAdvantages of Independent Logistics Consultants over System Integrators
“Leveraging broad supply chain experience from multiple industries, coupled with incisive analytics for precision system conceptual designs, and the capability to objectively examine and propose any design and system options – independent logistics consultants provide critical benefits that systems integrators cannot deliver, and continue to be the choice of supply chain executives for designing and […]
Continue ReadingPatrick Sedlak Elected to Material Handling Industry of America 2011 Industry Roundtable
Material Handling Industry of America (MHIA) announced the recent election of Patrick Sedlak to its 2011 MHIA Roundtable of Industry Leaders. In this capacity as an officer of the Roundtable, Patrick serves on the Material Handling Industry Board of Governors and the MHIA Directors At Large.
Continue ReadingRetrofit for Real ROI
Whether staying in your current building or moving to another, there are things you can do to maximize the return on your improvement investment. Lou Cerny shares his thoughts in this article on retrofitting distribution facilities.
Continue ReadingSubscribe For Insights
"*" indicates required fields